Pretransition state and apo structures of the filament-forming enzyme SgrAI elucidate mechanisms of activation and substrate specificity.
Shan, Z., Ghadirian, N., Lyumkis, D., & Horton, N. C.
Journal of Biological Chemistry.
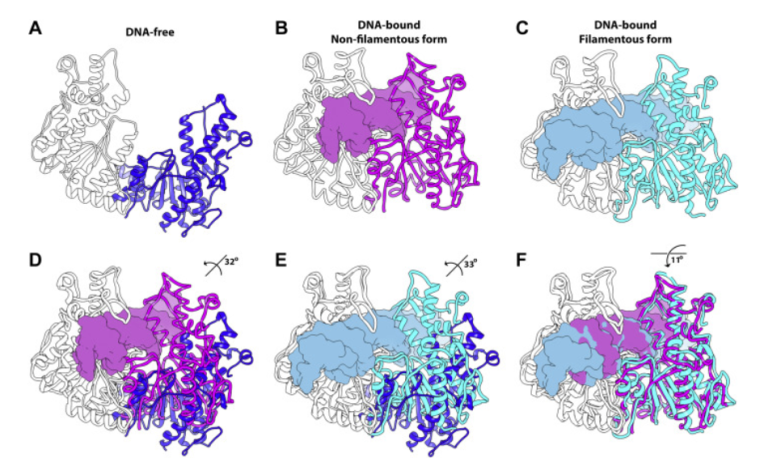
The Horton lab, in collaboration with the laboratory of Dmitry Lyumkis at The Salk Institute for Biological Sciences, recently determined a high-resolution structure of a filament-forming enzyme (SgrAI) which shows how enzyme filamentation activates this enzyme. The structure also provides new clues as to how filamentation expands the DNA sequence selectivity of the enzyme. Filament formation by enzymes is a newly recognized mechanism for controlling enzyme activity which is widespread and no doubt plays important roles in normal cellular functions and in diseases, many of which have yet to be discovered. This is the work of Niloofar Ghadarian, Ph.D. in Chemistry from the University of Arizona.
Link to paper: https://doi.org/10.1016/j.jbc.2022.101760